What happens to an electron when you put it into water? – Due to the negative charge of the electron and the permanent dipole moment of water molecules, the latter reorient themselves in a way that leads to a localization of the excess electron going along with a binding energy gain, a process that is called solvation. These processes play a crucial role whenever electrons are transferred between donors and acceptors in aqueous environments, which covers a plethora of reactions in biochemistry, e.g. photosynthesis, or corrosion. Despite its wide relevance, the initial processes of charge injection and transformation from a delocalized electron in the water conduction band to a trapped, localized electron could not be resolved so far. The very short lifetime of conduction band (CB) electrons even inhibited the measurement of the vertical binding energy of this important quantity.
Using femtosecond time-resolved two-photon photoemission, we are able to directly follow the “birth” of solvated electrons in amorphous solid water films on a Cu(111) single crystal surface. The Cu substrate serves as a source of electrons that are excited and subsequently transferred into the conduction band of ice. This way of injecting electrons into the water avoids disturbances that occur by ionizing the water molecules themselves or by dissolving additional ions. They relax to the band minimum (vertical binding energy, VBE = 800 meV) at a very high rate of 4 meV/fs. The trapping of electrons- and thus the formation of eS-occurs within 22 fs and results in a gain in binding energy of several 100 meV. We show that the trapping sites exist even before electronic excitation, i.e., they do not form by small polaron formation. Subsequently, they are stabilized by molecular reorientation on longer timescales (0.27 meV/fs). At the same time, the electronic coupling to the Cu(111) substrate leads to a decay of the solvated electron population with a time constant of 66 fs, i.e. significantly slower than the electron trapping process. This strongly suggests that the observed relaxation in the conduction band and the localization at preexisting sites is not significantly affected by population transfer to the metal. Our results may thus be representative even for bulk ice or, possibly, liquid water.
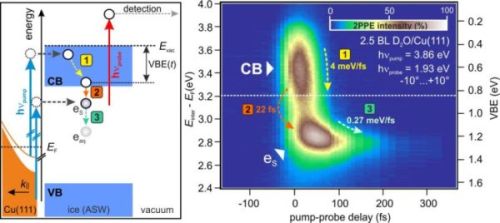
Energy and excitation scheme of electrons injected from the Cu(111) surface into an ASW layer (left). The corresponding experimental data (right) displays the pump-induced change in electronic population in the ASW layer during the first few hundred fs. Upon electron injection from the Cu substrate, electrons relax towards the CB minimum (1). Subsequently, solvated electrons are generated by population of trapping sites in the ASW (2). This is followed by energetic stabilization of the solvated electrons as the solvation shell evolves (3).
related publications
Julia Stähler, Jan-Christoph Deinert, Daniel Wegkamp, Sebastian Hagen, and Martin Wolf
Real time measurement of the vertical binding energy during the birth of a solvated electron
J. Am. Chem. Soc. 137 3520 (2015) (publisher version) (free full text)
press releases
Peter Hergersberg
Diving electrons / Ein Elektron auf Tauchgang